Propanoyl Chloride is a vital reagent in organic synthesis. It plays a key role in the acylation reactions of various compounds. According to recent industry reports, the global demand for propanoyl chloride has risen significantly. This is driven by its applications in pharmaceuticals and agrochemicals.
The synthesis of complex molecules often requires reliable intermediates. Propanoyl Chloride serves as a versatile building block. Its reactivity allows for the formation of esters, amides, and other derivatives. These reactions are crucial in drug development, where precision matters.
While its utility is evident, the handling of Propanoyl Chloride demands caution. It is corrosive and requires proper safety measures. Therefore, users must recognize its potential hazards. Awareness of best practices can prevent unintended reactions. This is essential for achieving successful outcomes in organic synthesis.

Propanoyl chloride, also known as propionic acid chloride, is an important reagent in organic synthesis. It has a molecular formula of C3H5ClO and a molecular weight of 92.53 g/mol. This colorless liquid has a pungent odor and is highly reactive. It typically boils at 104 °C and has a density of around 1.08 g/cm³. These properties make it useful for acylation reactions, particularly in the synthesis of esters and amides.
In terms of reactivity, propanoyl chloride readily reacts with alcohols and amines to form corresponding esters and amides. According to industry reports, acyl chlorides like propanoyl chloride have higher reactivity than carboxylic acids. This can speed up reaction times, but excessive reactivity can lead to side reactions. It’s essential to control reaction conditions carefully to avoid unwanted byproducts.
Safety data highlight that propanoyl chloride can be corrosive. Proper handling is crucial to avoid skin and respiratory irritation. The compound reacts violently with water, releasing hydrochloric acid. This poses additional risks during storage and usage. Attending to safety protocols is essential for any researcher or chemist working with this reagent.
| Property | Description |
|---|---|
| Chemical Formula | C3H5ClO |
| Molecular Weight | 92.53 g/mol |
| Appearance | Colorless liquid |
| Boiling Point | 102 °C |
| Melting Point | −56.5 °C |
| Solubility | Soluble in organic solvents |
| Uses in Organic Synthesis | Used in the synthesis of esters, amides, and in acylation reactions |
| Safety Precautions | Handle with care; may cause skin and eye irritation |
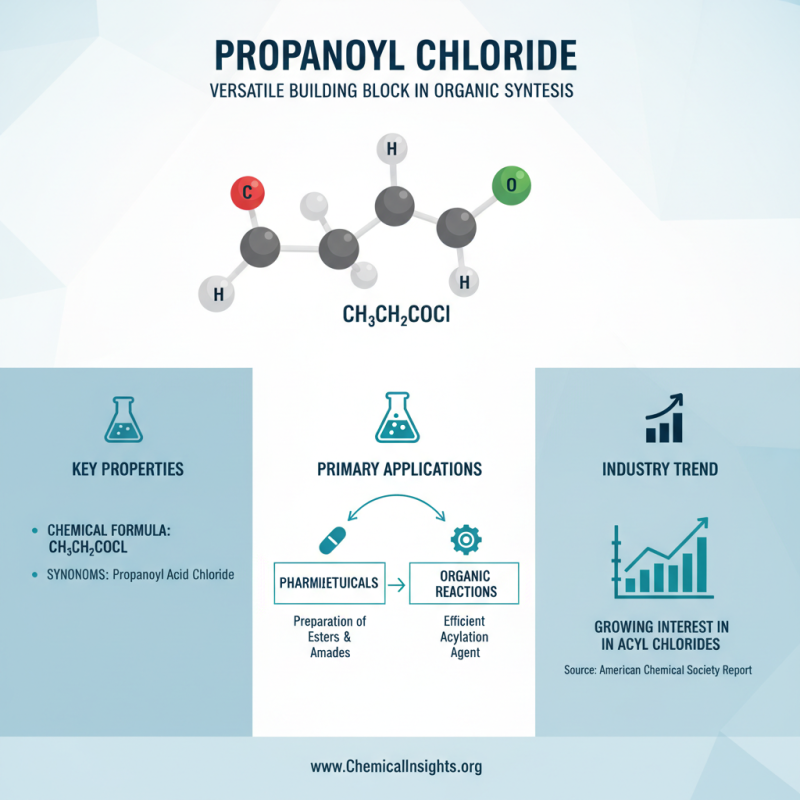
Propanoyl chloride, also known as propanoyl acid chloride, plays a significant role in organic synthesis. Its applications include the preparation of esters and amides, crucial in pharmaceuticals. A report from the American Chemical Society highlights a growing interest in acyl chlorides for their efficiency in various reactions.
One common method to synthesize propanoyl chloride is by reacting propanoic acid with thionyl chloride. This reaction typically has good yields, and conditions are manageable. It's essential to control temperature to avoid excess byproducts. Another approach involves using phosphorus pentachloride, which can also give decent yields but may require additional purification steps.
Tips: Always work in a fume hood. Propanoyl chloride emits irritating fumes. Protect your skin and eyes during the process. Also, use anhydrous solvents whenever possible; this helps in better yield and minimizes side reactions.
Using these methods may lead to some challenges. Byproducts can occur, and separation might be cumbersome. Do not rush the purification process. Experiment with reaction times and temperatures. This can significantly enhance your results. Always document your procedures for future reference.
Propanoyl chloride, also known as propionic acid chloride, is a versatile reagent in organic synthesis. Its reactions form essential intermediates in various chemical processes. A report from the American Chemical Society highlights its utility in acylation reactions, critical for producing esters and amides. This allows for more complex molecular architectures in pharmaceuticals and agrochemicals.
In organic synthesis, propanoyl chloride reacts readily with alcohols and amines. This leads to the formation of propanoyl esters and amides, compounds widely used in flavor and fragrance industries. According to a 2022 journal review, the reaction's efficiency can vary, often depending on the substrate's structure. Reaction conditions are crucial. Their optimization can significantly affect yield and purity.
Despite its advantages, using propanoyl chloride requires caution. The volatile nature and reactivity pose risks. Improper handling can lead to hazardous byproducts and safety concerns. As researchers navigate its applications, reflecting on these challenges ensures responsible practices. Continuous studies are needed to refine methods and address safety. This evolving landscape requires a mindful approach to harness propanoyl chloride's potential effectively.
Propanoyl chloride is an important reagent in organic synthesis. However, it requires careful handling due to its hazardous nature. It is classified as a corrosive substance. Proper safety measures are essential to prevent accidents. In 2019, the Chemical Safety Board reported a significant increase in safety incidents related to reactive chemicals. This highlights the need for strict adherence to safety protocols.
Personal protective equipment (PPE) is crucial when working with propanoyl chloride. Always wear gloves, goggles, and lab coats to minimize exposure. A fume hood is a must. Propanoyl chloride releases toxic fumes upon contact with moisture or water. In a laboratory setting, it is vital to keep it away from incompatible substances. Spills can create hazardous situations and require immediate attention.
Training is essential for all personnel handling this reagent. Regular safety drills can reinforce good practices. The National Institute for Occupational Safety and Health (NIOSH) advises having an emergency plan in place. Furthermore, proper storage conditions are key. Propanoyl chloride should be stored in tightly sealed containers in a cool, dry area. Reflecting on past incidents can help improve current safety standards. Mistakes teach important lessons in chemical safety.
Propanoyl chloride, also known as propionyl chloride, is vital in industrial chemistry. This compound is a key player in the manufacture of various pharmaceuticals and agrochemicals. According to a 2022 market analysis, the demand for propanoyl chloride is expected to grow at a steady rate of 3.5% per year. This consistent growth is largely driven by its applications in synthesizing esters and amides.
In the pharmaceutical industry, propanoyl chloride serves as an acylating agent. It helps create complex molecules needed for drug formulations. For example, it is used to synthesize analgesics. Yet, while beneficial, the handling of propanoyl chloride requires caution. Its corrosive nature can pose risks. Safety protocols must be strictly adhered to during its use.
Additionally, propanoyl chloride finds extensive use in producing flavoring agents. These compounds are essential in food chemistry. They contribute to the formulation of various food products. However, sourcing and environmental concerns are emerging. The industry must consider sustainable practices when utilizing this chemical. Balancing efficiency and safety presents a continuous challenge for manufacturers.